Q111: Synthesis
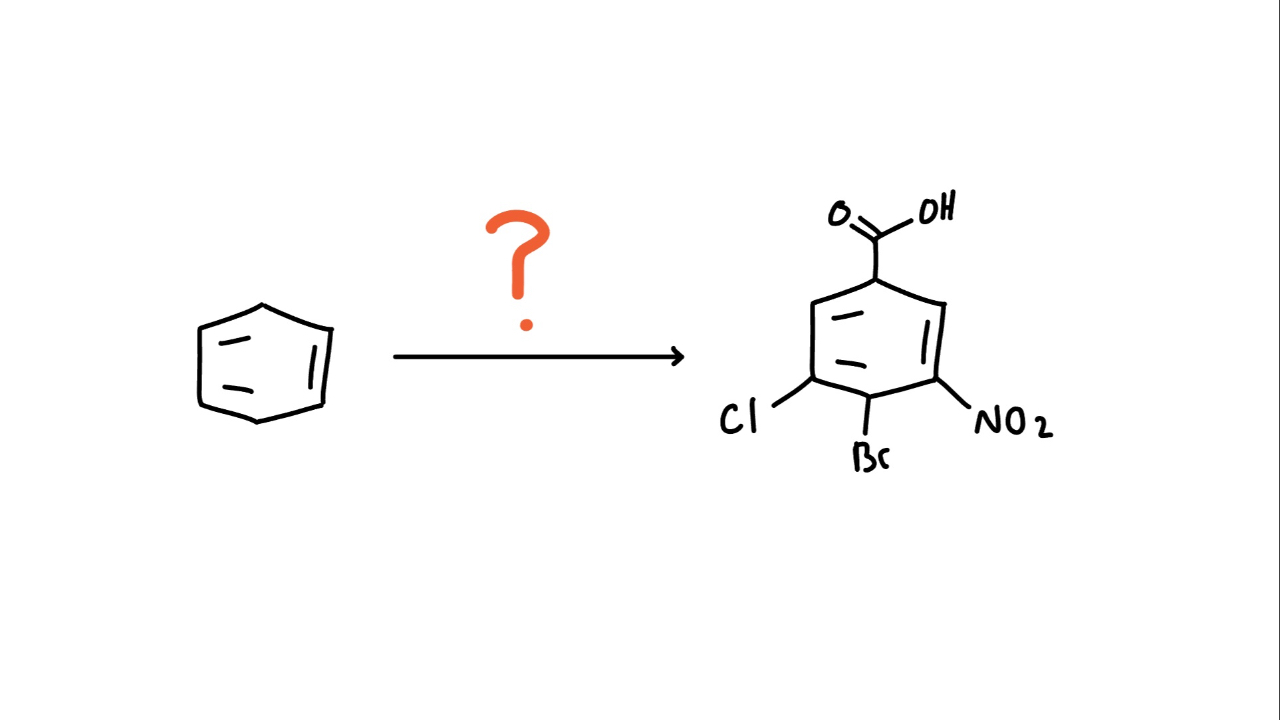
Provide an efficient synthesis to the reaction above!
Answer!
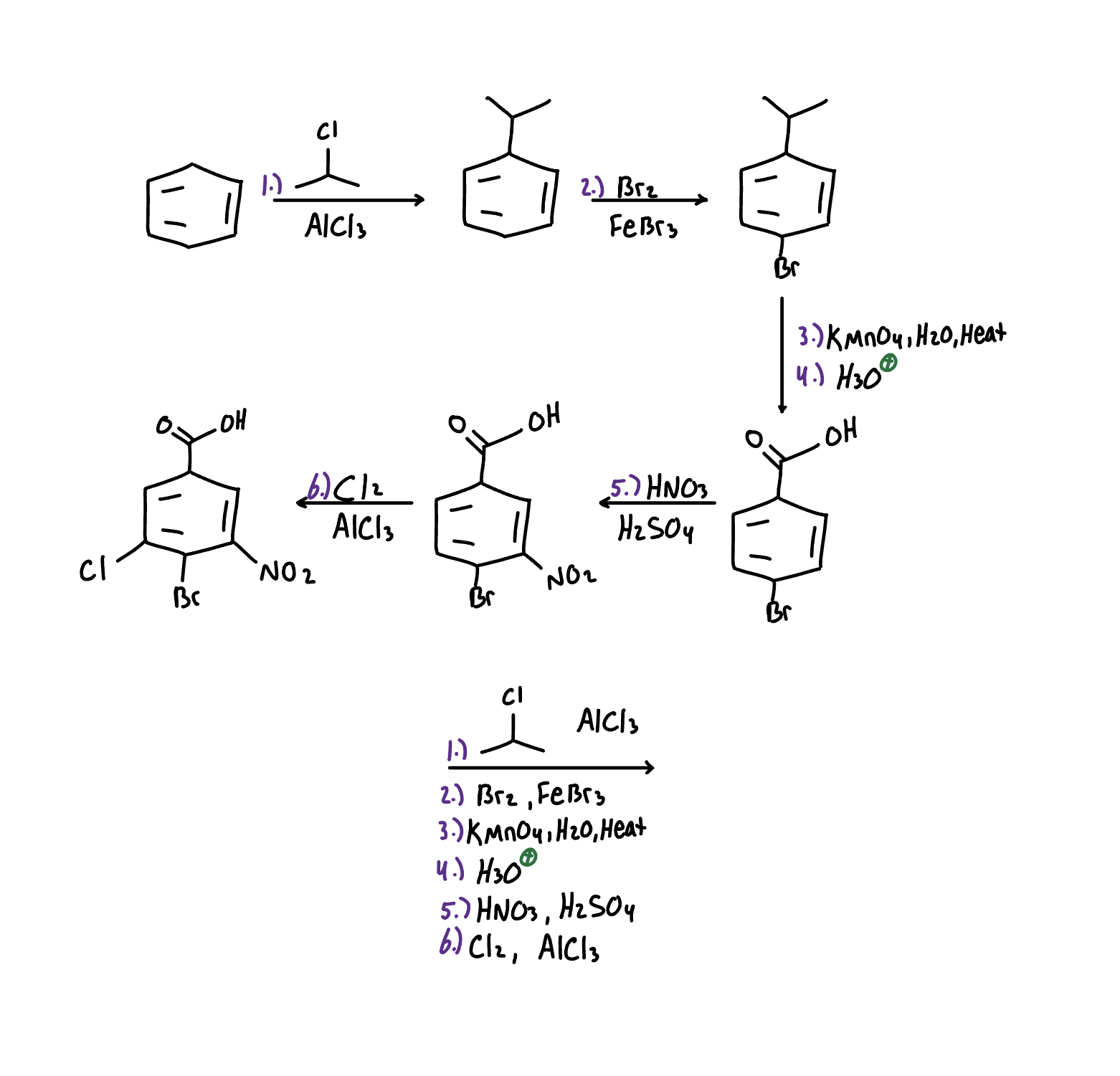
To start the reaction, we’ll add a donating group to the ring to boost its electron density. But which one?
We’ll start with a Friedel–Crafts alkylation to add an isopropyl group. This favors para substitution because of steric hindrance and it leaves a benzylic hydrogen, which we’ll need for oxidation later on!
Now that we have our activator, we can brominate the ring, adding bromine para to the isopropyl group.
But we’re not done yet. In our final product, we see a carboxylic acid. To get there, we’ll perform a benzylic oxidation to turn the benzylic carbon into a carboxylic acid. (KMn04, Heat, H20)
At this point, bromine becomes our strongest “activator,” so we’ll follow reactions directed by it.
Next, we add the nitro group through nitration which will be placed para to the bromine.
And finally, we finish with chlorination to add the chlorine group giving us the final product!
Need extra support this semester?
I partnered with Chemmunity to give you the best way to master Organic Chemistry. From on-demand lessons to weekly live tutoring sessions (led by me), it’s everything you need to feel confident this semester!


