Q108: What is the Relationship?
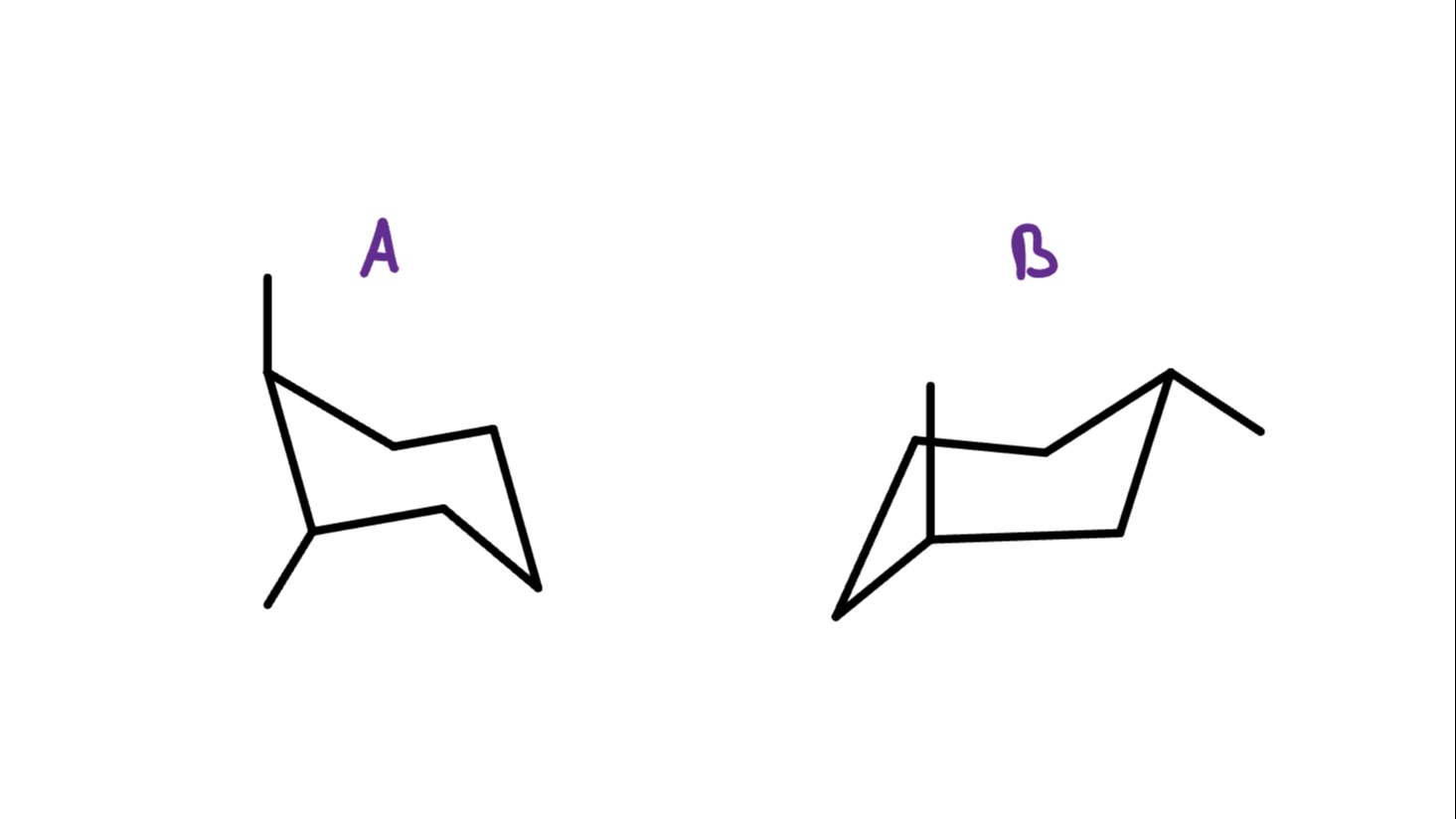
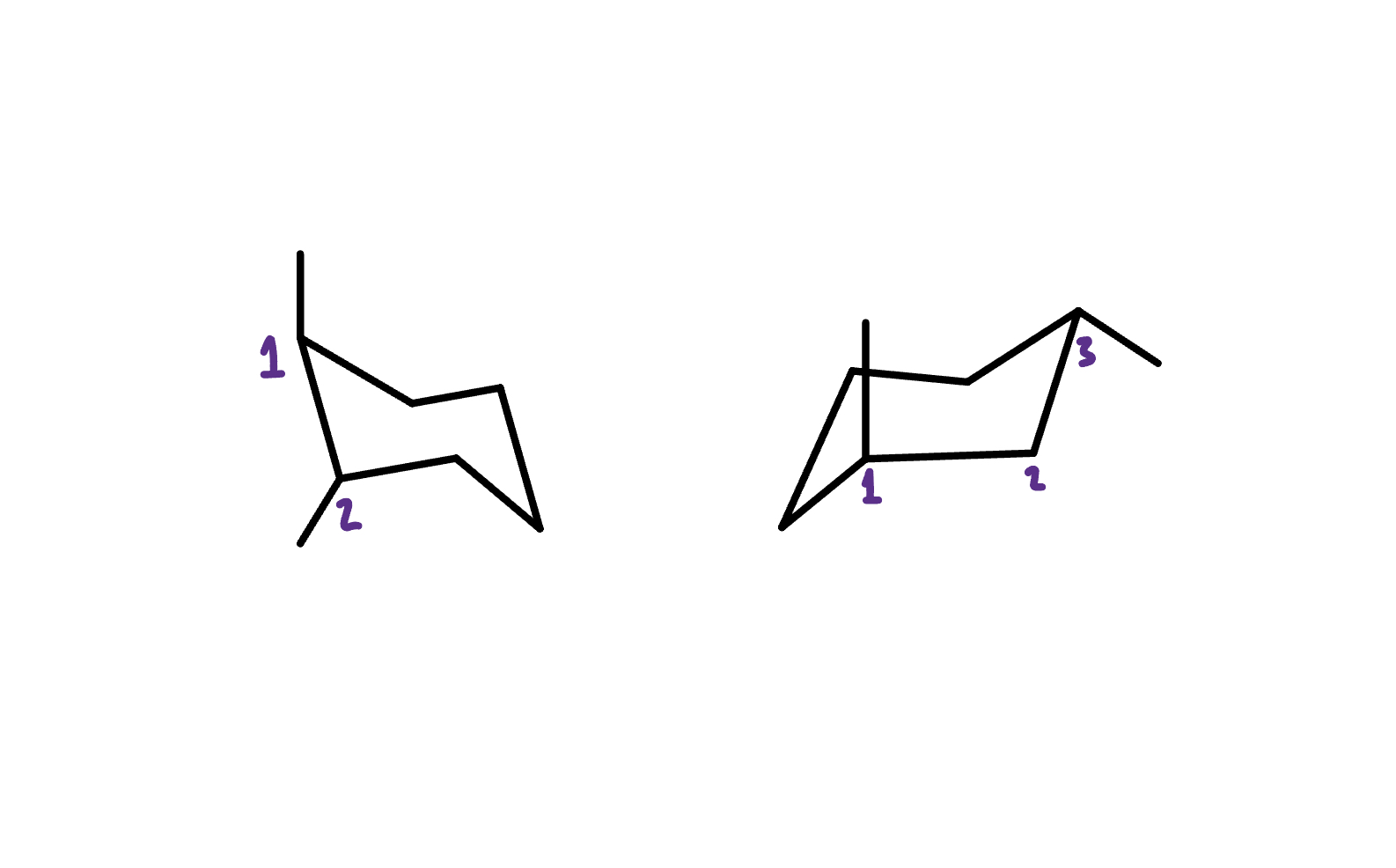
What is the Relationship between these two molecules?
A: Enantiomers
B: Constitutional Isomers
C: Diastereomers
Answer!
❌ Enantiomers
✅ Constitutional Isomers
❌ Diastereomers
The easiest way to tell is by looking at how far apart the substituents are in molecules A and B.
In molecule A, the methyl groups are right next to each other, but in molecule B, they’re separated by an extra carbon.
Since the molecules have the same molecular formula but different atomic connectivity, they’re constitutional isomers!
Need extra support this semester?
I partnered with Chemmunity to give you the best way to master Organic Chemistry. From on-demand lessons to weekly live tutoring sessions (led by me), it’s everything you need to feel confident this semester!


